Promepla is a new member of the Collège Français de Métrologie (CFM).
The CFM is a reference in the field of metrology for all those involved in measurement, helping its network members to optimise their practices. Why did we decide to join the CFM? Our mission is to design and develop components and assemblies for medical devices on behalf of our customers. We aim to constantly innovate using new techniques, adding value to the product, both in terms of its composition and its use, while complying with current standards and regulations. Product quality, safety and traceability are fundamental industrial principles for our company. Metrology is present at every stage of our processes, enabling us to design the conditions for observing a phenomenon, to build and qualify our instruments, and to determine whether the results obtained are significant in order to guarantee the impeccable quality of the products we manufacture. Our commitment to a high standard quality policy has led us to join the CFM in order to optimise our measurement processes, broaden our skills, keep abreast of developments, and anticipate changes in metrology and requirements.

LATEST NEWS
We are pleased to present our new company websites:
A. Hopf Medical Tubing Members of the Promepla group since 2022 and 2023, our two companies now have two dedicated websites. The products manufactured by each of them are presented and the content also reflects their values and expertise, with a design in line with the Promepla brand image. Feel free to browse them to get to know us better.

Promepla S.A.M. has received its CE marking certificate for almost 90 of its products under Regulation (EU) 2017/745 on medical devices (MDR).
The certificate, issued by the Italian notified body IMQ S.p.A (0051), covers class Is (sterile) and class IIa devices, divided into 5 families: This CE mark confirms Promepla’s position as a leading player in the field of medical devices.
As a reminder, medical devices (MD) cannot be released in Europe without CE marking. All MD manufacturers must implement a quality management system within their organisation. In July 2024, Promepla S.A.M. renewed its ISO 13485 certificate, a guarantee of compliance and continuous improvement. In order to affix the CE mark, an MD manufacturer must also compile technical documentation (TD) that meets the requirements of European regulations (MDR) and demonstrates the safety and performance of its products.

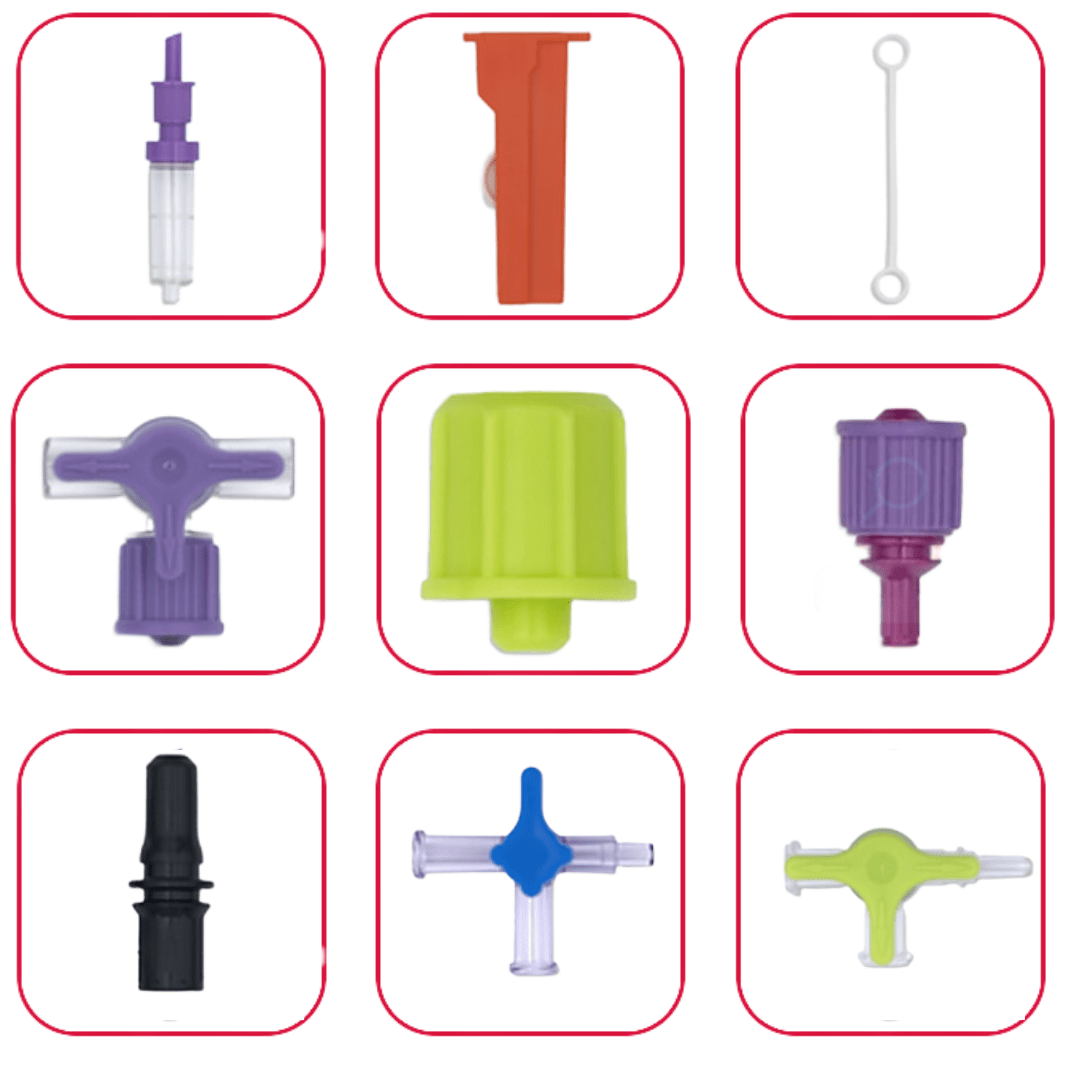
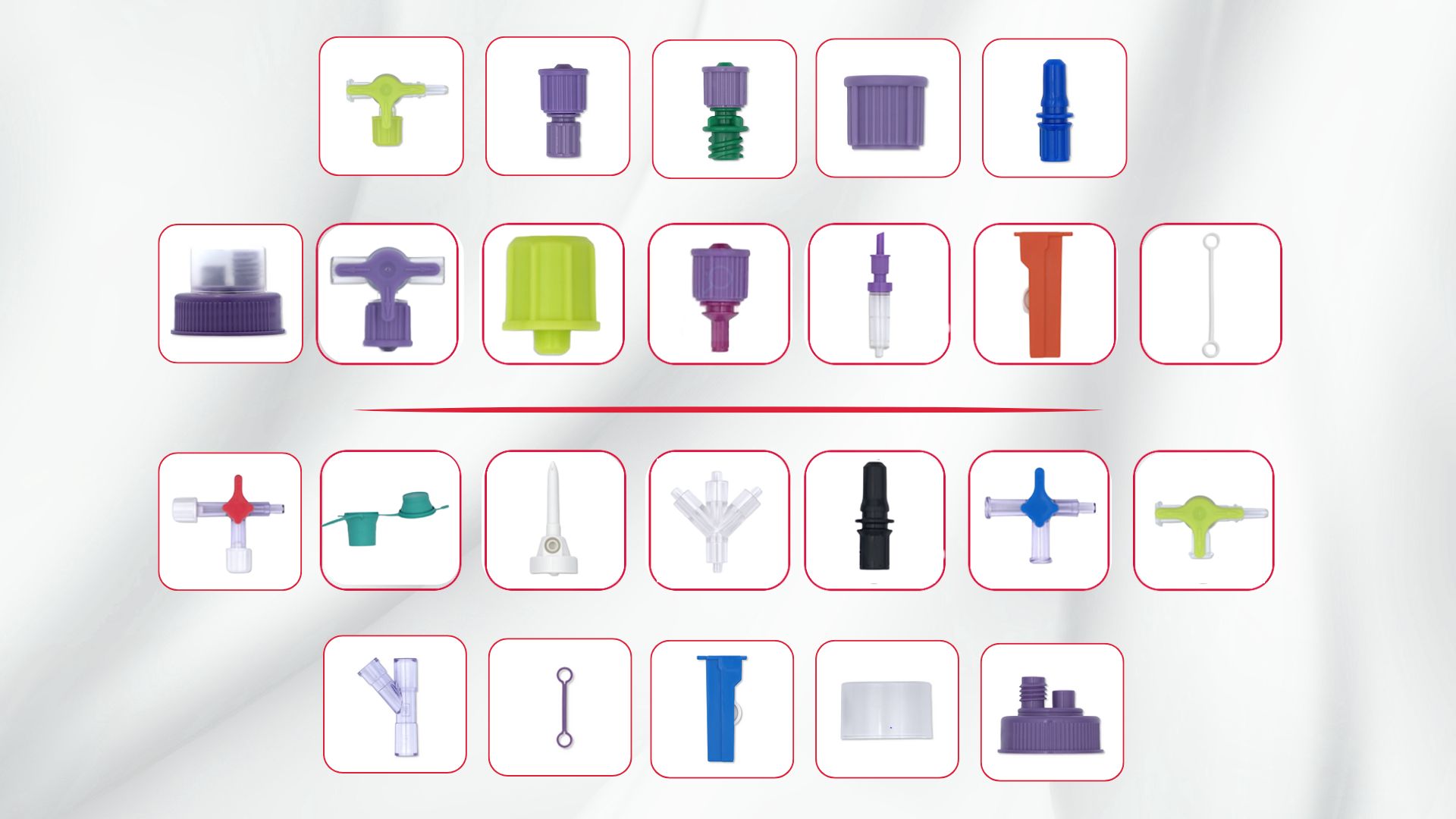
We are pleased to introduce a new section in our components catalog, the A. Hopf dedicated section. These injection molded parts are manufactured exclusively in Germany and include:
Please contact us if you would like to get more information about these components: contact@promepla.com About A. Hopf GmbH
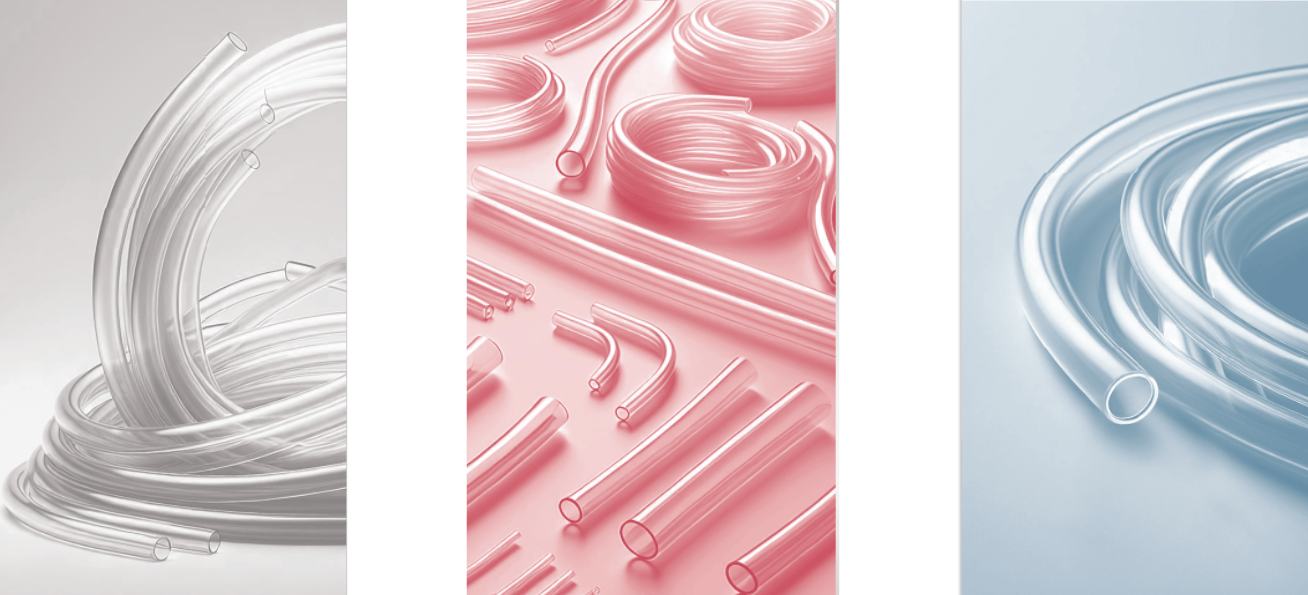
The PVC used in these new flexible medical hoses is manufactured using: Consequently, the carbon footprint of this PVC is significantly lower than that of conventionally produced PVC. These new medical tubes have the same quality and properties as our conventionally produced PVC products.
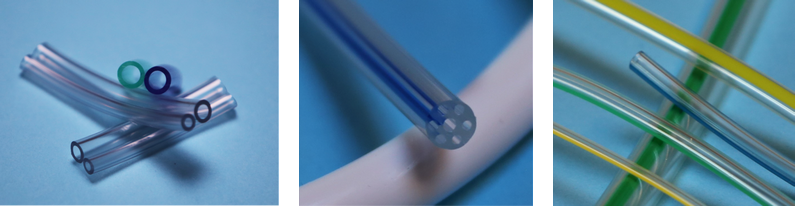
Please contact us if you would like to get a quote for these medical hoses: contact@promepla.com About Medical Tubing Based in Le Bousquet d’Orb, in the French Hérault region, Medical Tubing offers its customers 30 years of experience in thermoplastic extrusion. The company serves all medical markets with tubes of all sizes, including multi-layer and multi-lumen options. It also offers compounding of multiple resins for greater responsiveness. Since 2023, Medical Tubing has been part of the Promepla Group, a full-service provider and contract manufacturer for medical devices and biopharmaceutical solutions. http://www.medical-tubing.com/
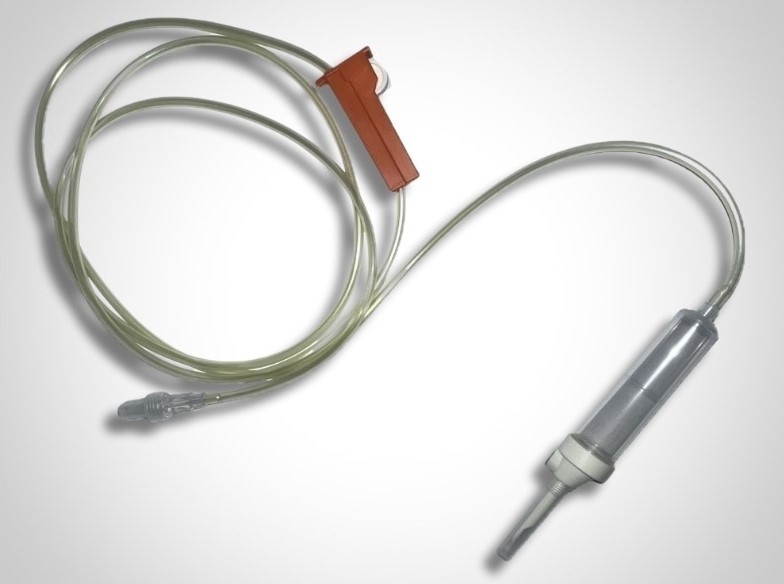
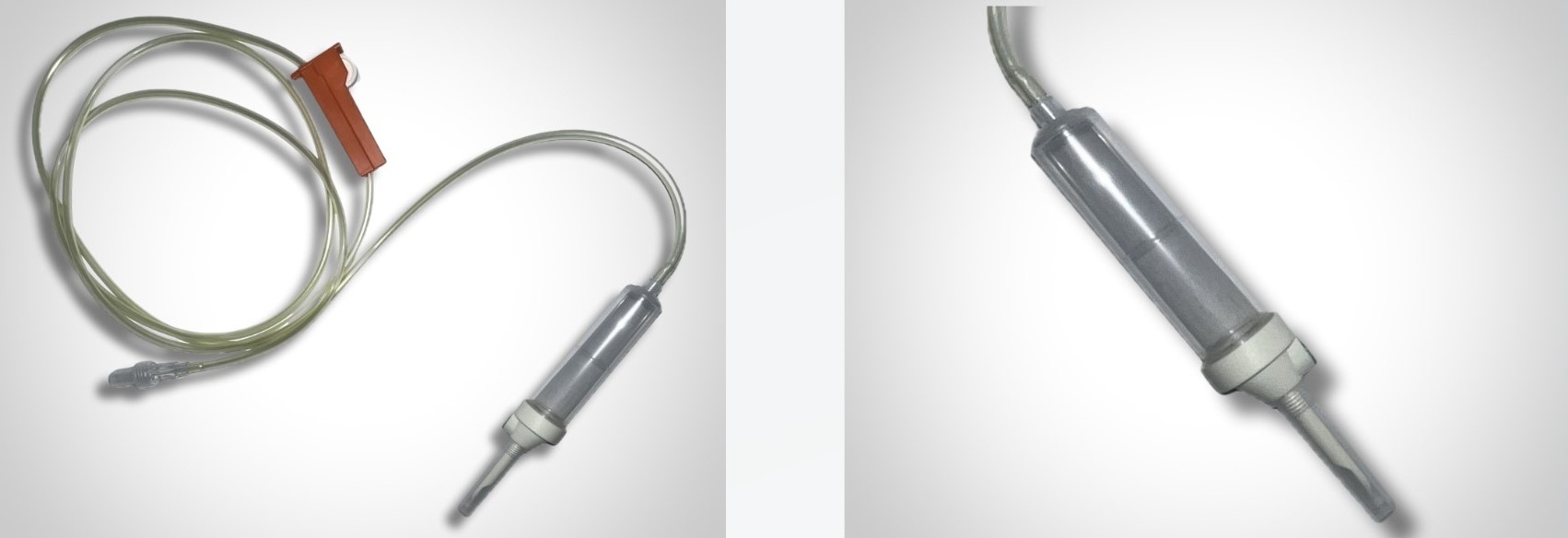
A. Hopf GmbH, a pioneer in the development of BPA-free components, now features a PVC-free IV set.
This set consists of: Regarding the various connections (since PP can be difficult to bond and requires special treatment): Do not hesitate to come back to us for more information on this PVC-free intravenous set: contact@promepla.com. About A. Hopf GmbH A. Hopf Kunststoffverarbeitung GmbH is a high-precision injection molder and mold manufacturer for medical components. Hopf products are characterized by high quality, excellence and reliability and are manufactured exclusively in Germany. Since 2022, Hopf has been part of the Promepla Group, a full-service provider and contract manufacturer for medical devices and biopharmaceutical solutions.
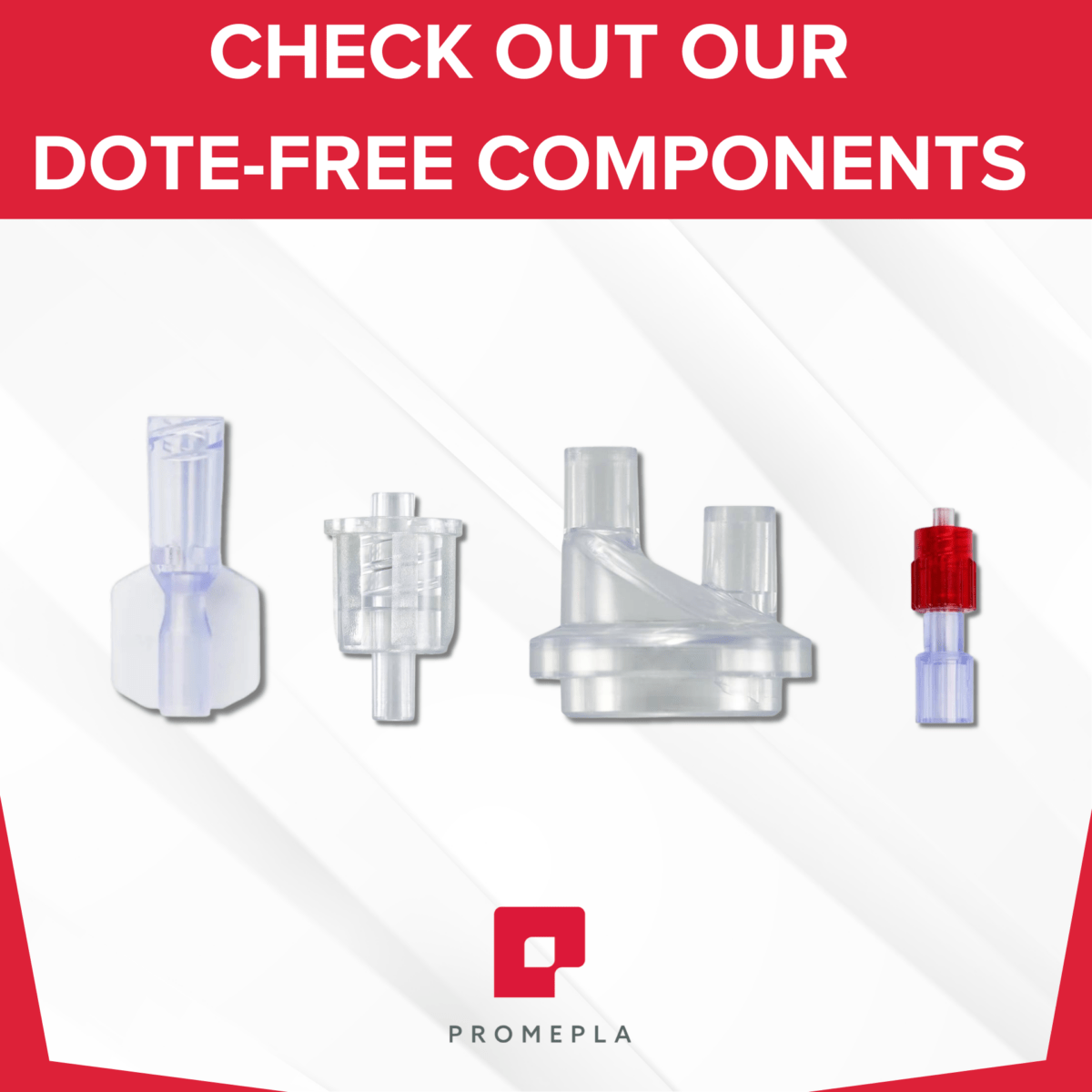
The DOTE substance (2-ethylhexyl 10-ethyl-4,4-dioctyl-7-oxo-8-oxa-3,5-dithia-4- stannatetradecanoate), a thermal stabilizer commonly used in rigid PVC, was added in 2022 to Annex XIV of the European Union (EU) REACH Authorisation List due to its “toxic for reproduction” intrinsic property (Article 57c). As a result, DOTE should no longer be used in medical device components from May 1st 2025. Do any of your PVC products still contain DOTE?
We are at your disposal to help you source alternative DOTE-free components for medical devices, do not hesitate to contact us: contact@promepla.com.
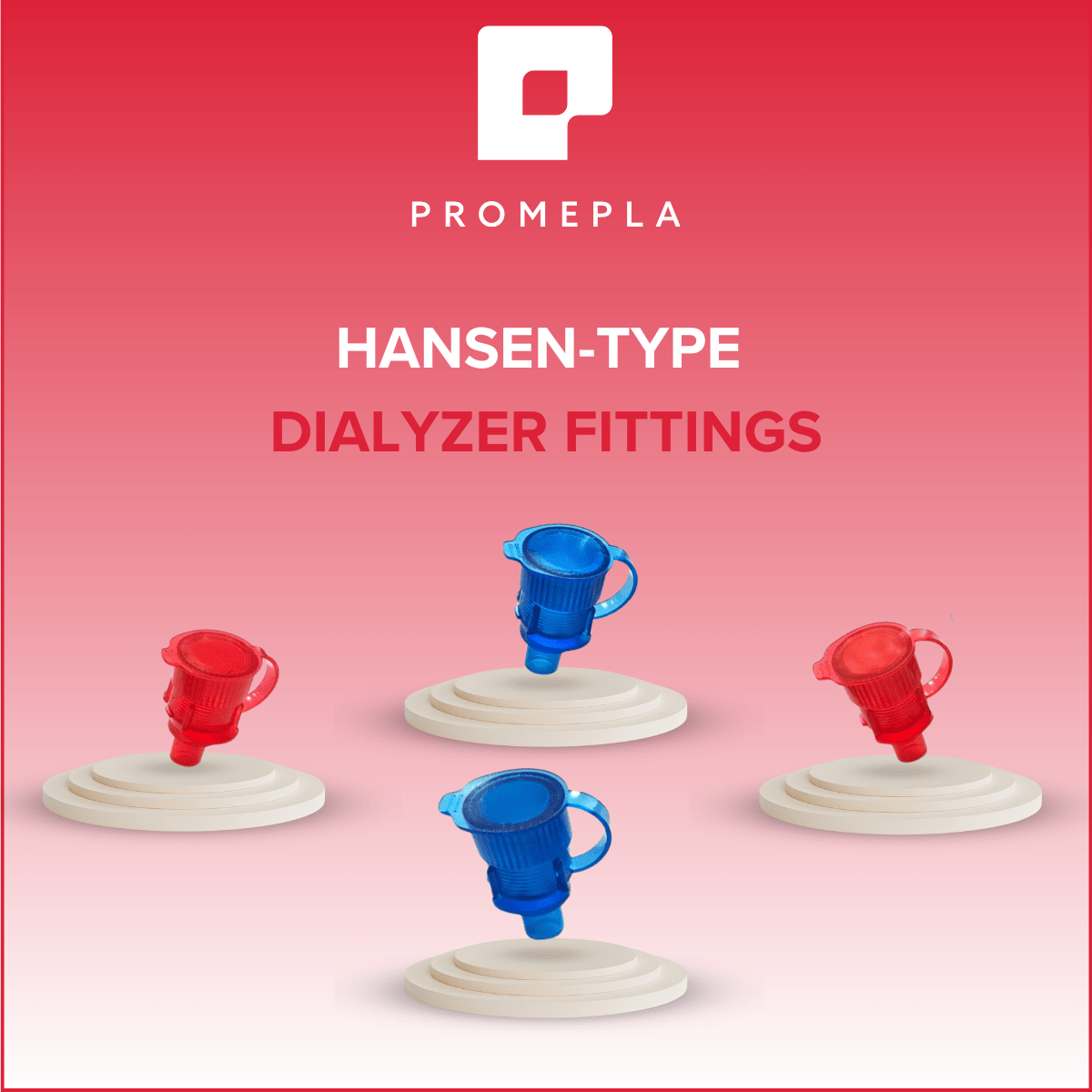
Are you looking for a coloured Hansen-type dialyzer connector? We have red and blue references back in stock!
To order them or ask for samples:
We are happy to announce that we have renewed our ISO 13485:2016 certification for 3 years. This is the result of the hard work and daily commitment of our teams.

We are expanding our range of services and industrial capabilities with the acquisition of the French company Medical Tubing.
Based in Le Bousquet d’Orb, in the Hérault region, Medical Tubing offers its customers 30 years of experience in thermoplastic extrusion. The company serves all medical markets with tubes of all sizes, multi-layer and multi-lumen. It also offers compounding of multiple resins for greater responsiveness. With Medical Tubing, we complete our offering as a solutions integrator for all single-use medical device and biopharmaceutical development projects. Following the acquisition in 2022 of A. Hopf GmbH, a German company based in Cadolzburg, we now cover the entire value chain for single-use life science devices, with more than 3,300 sqm of ISO 8 cleanroom space : These investments in France and Europe in the medical sector enable us to offer European and global medical device players a single point of entry for their projects, reducing development times and reducing costs and integrating the challenges of industrialisation and marketing from the outset. http://www.medical-tubing.com/