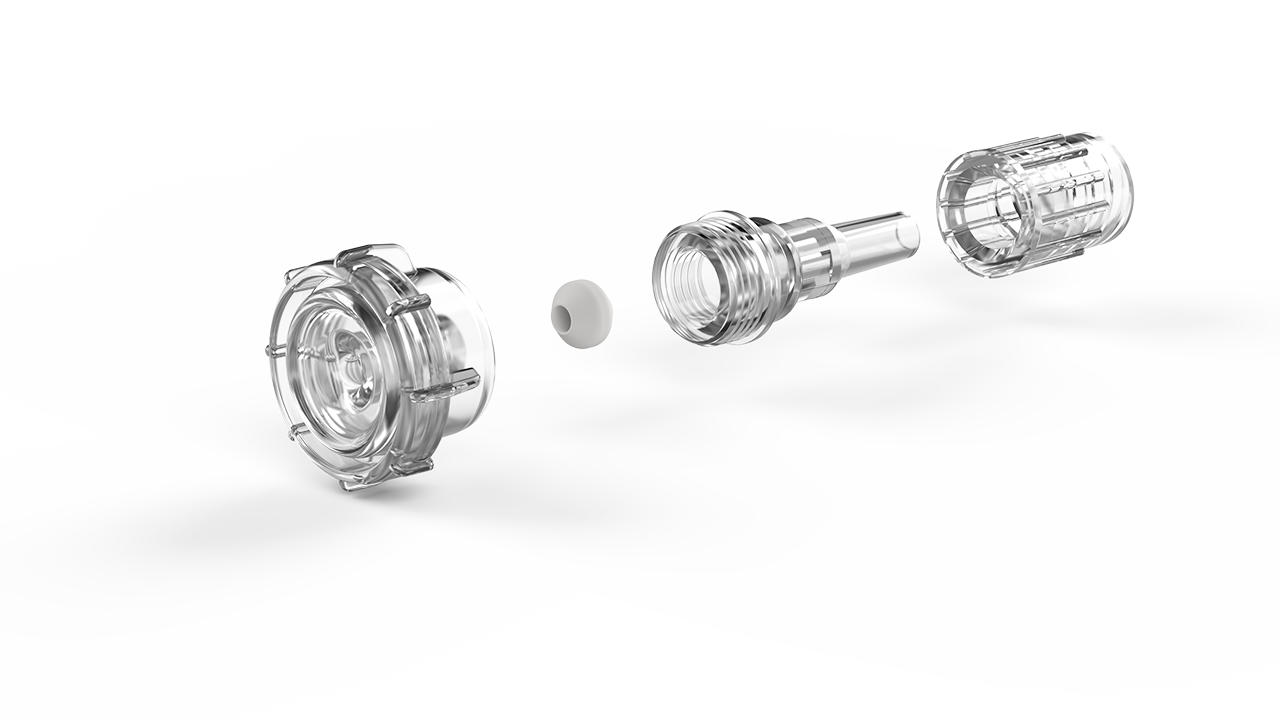
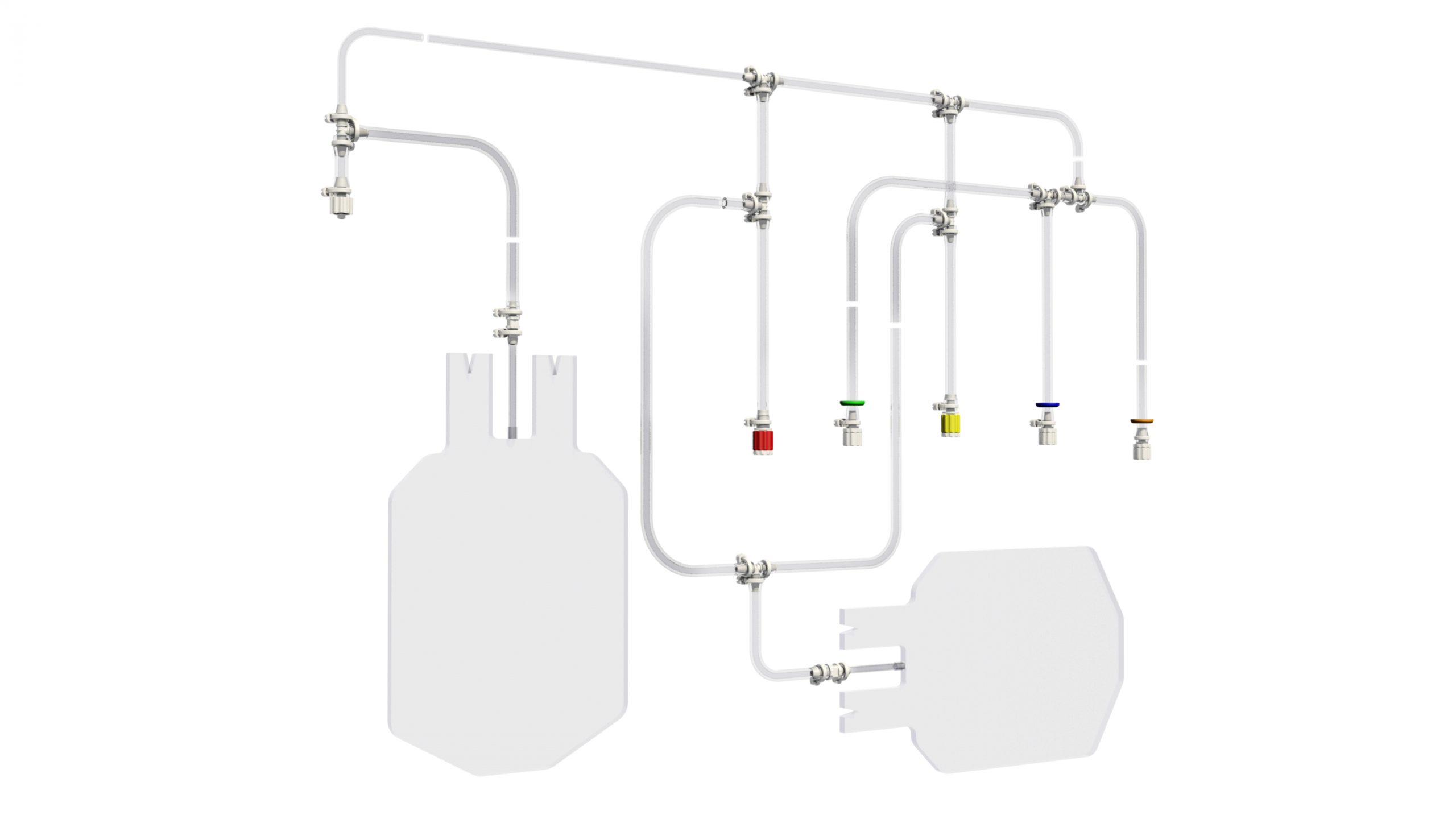
How many times have you been wondering how to partner adequately the development and manufacturing of your next-generation single-use device? Too many, and too often for sure. This needs a strong support from a reliable company you cannot find easily. Therefore, your development might then be at stake but slowing down this process is not an option you can consider. You mandatorily need to reach and meet this partner who will provide you the keys to the right choice and will be available to help you quickly. Within this period when innovation is to be accelerated whilist being driven by the need of securing any process, PROMEPLA provides a best-in-class support from A to Z for your device to be considered. For the latest 50 years, PROMEPLA has been specialized in designing, developing and manufacturing medical and biopharmaceuticals devices. Up to CE marking and MDR activities support coming, QA and R1 specs reached, PROMEPLA is the right partner you can find for the assemblies and devices to be marketed quickly.Innovating through materials choice, assembling processes, and applications knowledge are some keys to success for your single-use device to be marketed securely and complying with any of the specifications. As a solutions provider, PROMEPLA will use their unique integrated approach to facilitate the compliance of your device and its feasibility through 3 areas of expertise:
AREAS OF EXPERTISE
Supporting your developments and manufacturing: meet the adequate partner in single-use devices