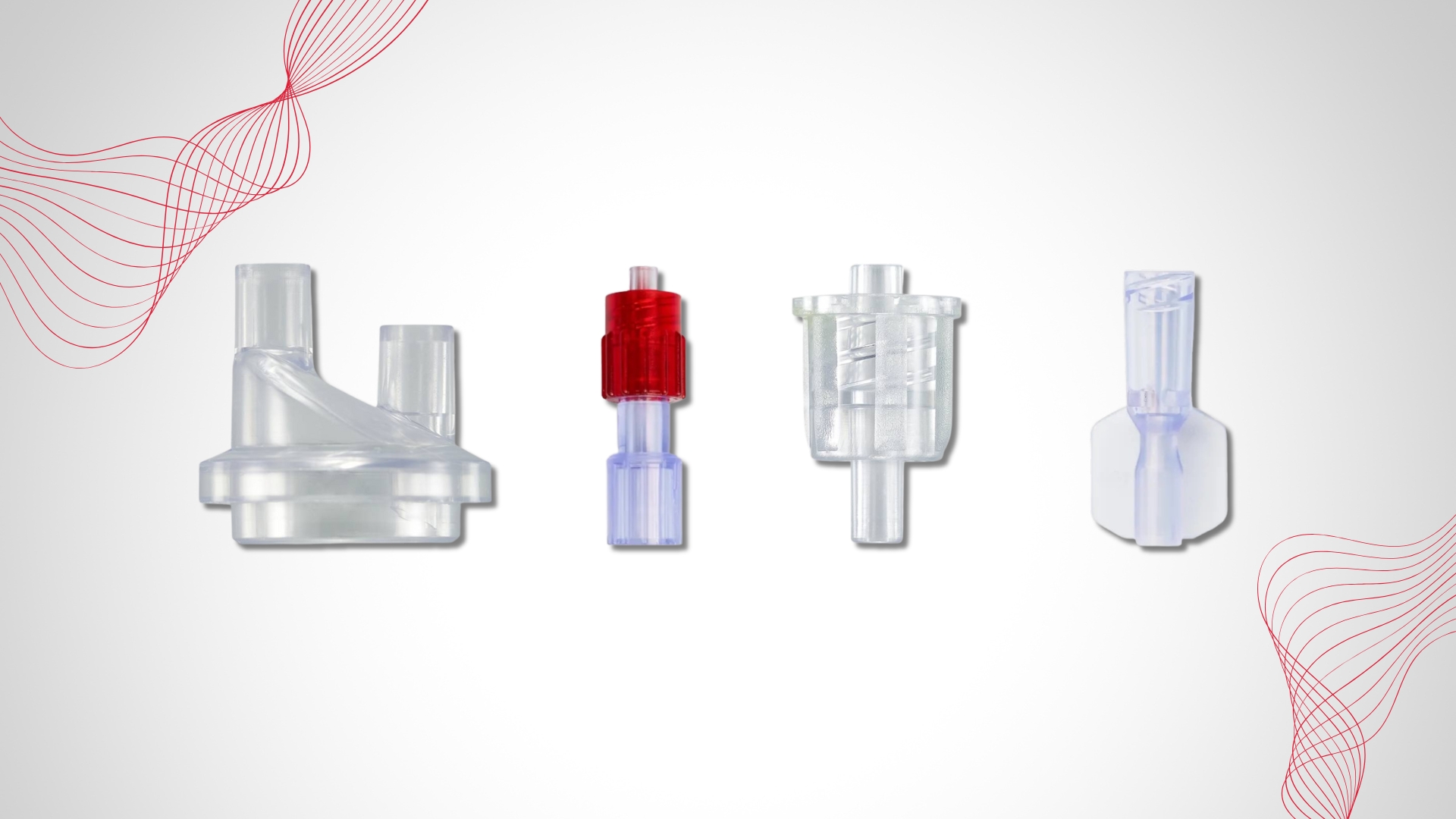
DOTE-free components
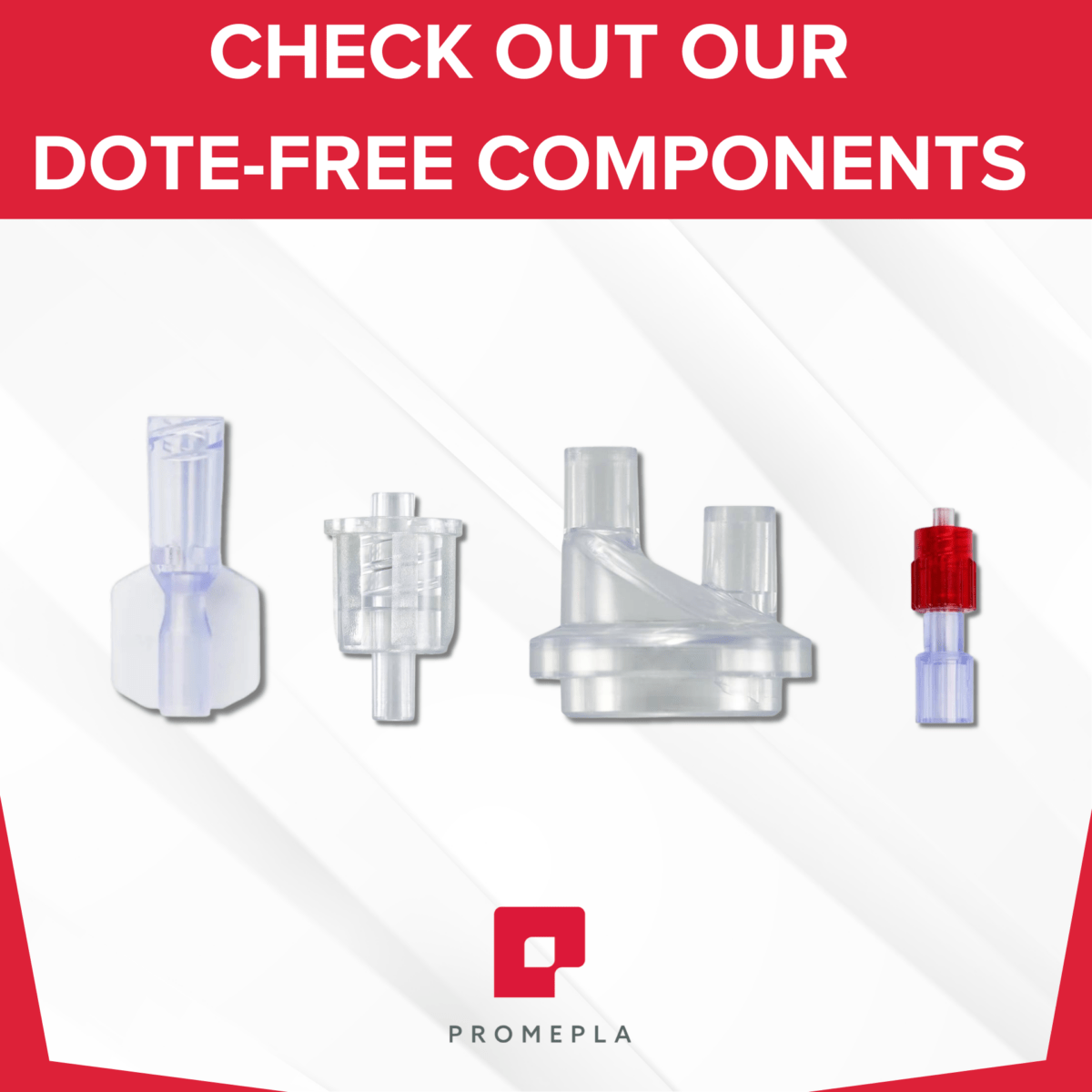
The DOTE substance (2-ethylhexyl 10-ethyl-4,4-dioctyl-7-oxo-8-oxa-3,5-dithia-4- stannatetradecanoate), a thermal stabilizer commonly used in rigid PVC, was added in 2022 to Annex XIV of the European Union (EU) REACH Authorisation List due to its “toxic for reproduction” intrinsic property (Article 57c). As a result, DOTE should no longer be used in medical device components from May 1st 2025. Do any of your PVC products still contain DOTE?
We are at your disposal to help you source alternative DOTE-free components for medical devices, do not hesitate to contact us: contact@promepla.com.